Orthocell at forefront of growing market opportunity in regenerative musculoskeletal treatment
Orthocell Ltd (ASX:OCC) is confident that its unique regenerative medicine product portfolio can address growing market opportunities in the treatment of complex musculoskeletal disorders.
The company is pursuing a total addressable market for CelGro® and Ortho-ATI® of US$10 billion and US$7.7 billion respectively – representing a combined US$17.7 billion per annum.
This market is driven by the rising rate of musculoskeletal disorders and demand for efficient and cost-effective treatments.
CelGro® nerve repair[hhmc]
The companys CellGro collagen medical device is designed to augment surgical repair of soft tissue and represents a breakthrough is soft tissue reconstruction.
It has multiple applications in nerve, tendon and bone repair which have been demonstrated as having superior clinical performance when compared to current market leading product.
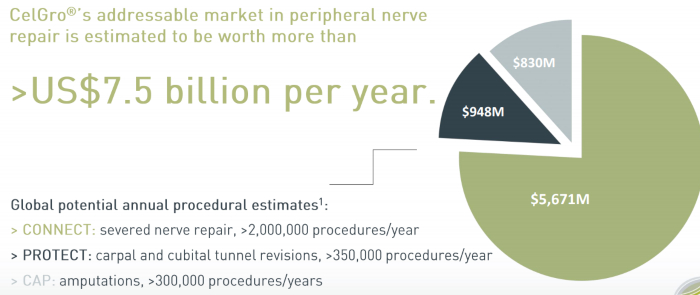
CelGro nerve repair market opportunity.
Addressable market opportunities[hhmc]
CelGro has significant global commercial potential in its existing addressable markets as well as much wider applications in general surgical and soft tissue reconstruction applications.
Nerve regeneration has a US$7.5 billion addressable market, tendon and ligaments have a US$1.4 billion addressable market and bone regeneration has a US$1 billion addressable market.
The company is also executing a strategy to engage a global partner to manage the distribution and marketing of CelGro for dental bone and soft tissue repair procedures.
In addition, the company has identified that there has been very little innovation in the biologics membrane market resulting in a lack of product differentiation which represents an addressable market of more than US$1 billion per annum.
Pathway to US market[hhmc]
Orthocell has achieved initial EU approval for the product and with the safety and efficiency of the CelGro nerve repair product established, the company is now focused on executing its regulatory program to gain approval in the US.
The company is focused on the US 510(k) study which aims to support an evaluation of substantial equivalence to an approved nerve repair device (meeting the requirements of the US 510(k) predicate product regulatory pathway).
The US FDA pre-submission meeting has been completed, and ethics approval granted.
Surgical procedures will commence in quarter four 2020 with the target for the final data read out of quarter four 2021.

The addressable market for Ortho-ATI is estimated at greater than US$7.7 billion per annum.
Ortho-ATI® therapy[hhmc]
The Ortho-ATI cell therapy for tendon regeneration is an injectable clinical stage cellular therapy for treatment of chronic tendon injuries. It can be utilised at multiple tendon sites including shoulder, elbow, hip, hamstring and achilles.
The therapy addressed an unmet clinical need for a safe, effective, and non-surgical option and is the first injectable cellular therapy in orthopaedics for tendon regeneration.
Ortho-ATI also has extensive clinical validation with more than 690 patients treated to date.
Multiple addressable markets[hhmc]
Ortho-ATI is at the forefront of a large and growing market opportunity where the addressable market is estimated to be greater than US$7.7 billion per annum.
This included tennis elbow Read More – Source
[contf]
[contfnew]

Proactiveinvestors
[contfnewc]
[contfnewc]




