MGC Pharmaceuticals leads the way in phytocannabinoid derived medicines
MGC Pharmaceuticals Ltd (ASX:MXC) is leading the way in phytocannabinoid derived medicines, using its nature to medicine strategy for delivery to patients.
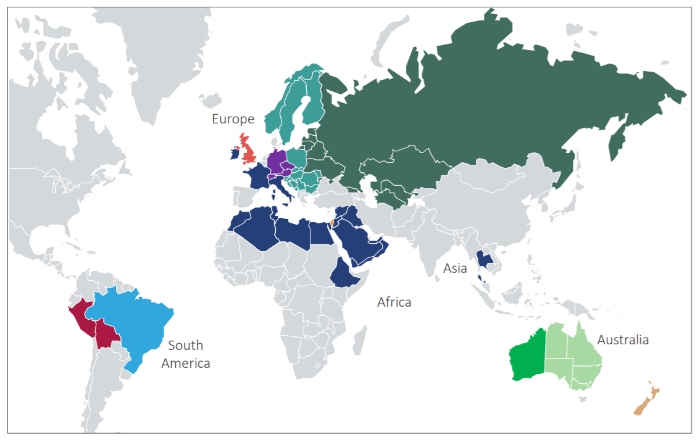
The European-based, vertically integrated company supplies EU-GMP phytocannabinoid products to patients, with products already available in Australia, New Zealand, the United Kingdom, Ireland and Brazil.
The company has two leading cannabinoid medicinal products in late-stage development – CannEpil®, an oral oil solution treatment for refractory epilepsy and CogniCann®, an oral spray for relief of dementia symptoms.
With facilities in Australia, Slovenia and the Czech Republic, MGC Pharmas mission is to build an innovative company that provides affordable phytocannabinoid medicines for targeted global markets and patients.
The company has an extensive network in place providing access to hospitals, pharmacies, and research institutions.
MGC Pharma partners and collaborates with academia worldwide such as RMIT University, Epilepsy Action Australia, Lenis and the Medicinal Cannabis Council to conduct extensive research and develop the highest quality products for patients.
Co-founder and MD Roby Zomer told Proactive the existence of compelling data in the areas of Alzheimers and Dementia led the company to believe it had a formulation of value, intersected with a large global need.
“The ageing population the world over needs access to solutions that can combat the large incidences of these indications and there are several benefits to phytocannabinoids that can impact more than just the core indication, giving us a product that can potentially tap a massive market with ever-growing demand.
“We are the sharp edge of an industry that we believe is going to change the world of pharma for the better.
“We see the evidence of this every day in our CannEpil patients, are encouraged by incidental and clinical data emerging on CogniCann, and are developing future clinically supported pipeline products to keep us ahead of the market for the next ten years.”
Potential share price and market share growth[hhmc]
He said the potential growth in its share price and market share were massive.
“We are making what we think are all the right steps to take advantage of our current position to learn how to work correctly in this ever-changing global dynamic."
MGC Pharma anticipates market growth will occur mainly across Europe as it sees the change in attitude and legislation across the continent including the Czech Republic, Slovenia, Greece, Macedonia and Germany.
The secondary market, Australia, is where the company is involved in encouraging legislation and creating an environment that promotes the growth of the industry.
MGC Pharma is seeing changes in regulation in Australia for the Cannabis-based medicine industry and aims to provide affordable medication and products to consumers.
COVID-19 impacts[hhmc]
While the COVID-19 pandemic has been incredibly challenging for the company, Zomer said it had also been an opportunity to learn more about themselves as a business and perhaps more importantly, how the business dealt with adversity.
“COVID pushed our creativity, our ingenuity, our limits and horizons as far as what can be achieved with technology and we believe we will be seeing the impact of this on both the supply chain and future logistics for years to come, though we welcome some sort of return to normal.”
June saw the company achieve several milestones such as surpassing the 3,250-prescription mark, selling its Nutraceuticals business to US-based CBD and hemp wellness company Onassis Holdings Corp and being awarded a three-year GMP licence from EU authorities to continue producing pharma-grade cannabinoid medicines and products.
However, Zomer said while it had been busy, he said it represented what the company was constantly engaged in – finding ways to maximise its market access, entering into new and potentially lucrative markets and tightening its focus by divesting assets which were no longer part of the core business.
“In many ways, June is just when the public saw some of the things that have been going on for months, if not years.
“This 3-year renewal in Slovenia represents a few things – it is a vote of confidence from the strictest GMP authority in the EU.
“It means we are taking the steps we need to be as a company – building relationships of value in the regions where we operate so we are not being viewed as part of a trend or fly-by-night operation by the regulatory authorities.”
Grant of Import Licence[hhmc]
Adding to its list of achievements, the multinational biopharmaceutical company received the grant of an Import Licence by the Australian Office of Drug Control (ODC) on June 8, allowing it to import any of its schedule-4 and schedule-8 medicinal cannabis products into Australia.
The company believes the granting of this licence significantly decreases logistics and handling fees and supports its business model of providing high-quality affordable medicinal cannabis products to patients.
MGC Pharma will now bulk import its medicinal cannabis products for storage, distribution and saleRead More – Source
[contf]
[contfnew]

Proactiveinvestors
[contfnewc]
[contfnewc]




