Kazia Therapeutics interim data from clinical studies reinforces positive efficacy signals
Kazia Therapeutics Limited (ASX KZA) (NASDAQ: KZIA) has shared poster presentations of interim data from its ongoing phase II study of paxalisib (formerly GDC-0084) in glioblastoma and from the phase I study of Cantrixil in ovarian cancer.
paxalisib phase II study[hhmc]
Previous paxalisib data presented at ASCO (American Society of Clinical Oncology) was based on Stage 1 (n=9) of the ongoing phase II study in glioblastoma, the most common and aggressive form of primary brain cancer.
This interim analysis at AACR (American Association for Cancer Research) includes all patients in the study (n=30), and therefore provides a more robust and substantial data set (poster link).
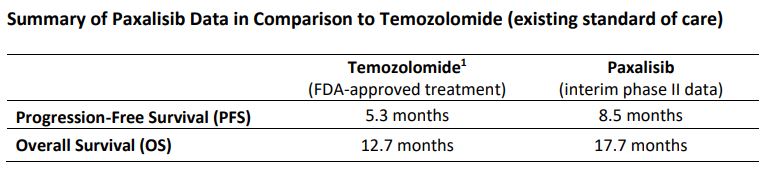
The progression-free survival (PFS) for paxalisib is now 8.5 months, versus 8.4 months in the previous analysis.
Paxalisib overall survival (OS) remains at 17.7 months, in line with ASCO data.
A separate poster on the investigator-initiated study of paxalisib in combination with radiotherapy is presented by clinicians at Memorial Sloan Kettering Cancer Center in New York.
It noted a robust response in the first treated patient.
Cantrixil phase I study[hhmc]
Cantrixil data shows one complete response (CR) to treatment, meaning no measurable disease, and two partial responses (PR), for an overall response rate of 19% (3 / 16 evaluable patients).
Increasing confidence in clinical programs[hhmc]
Kazia CEO Dr James Garner said: “The data summarized in these posters help to strengthen our confidence in both our clinical programs.
“As paxalisib moves towards commencement of the GBM AGILE pivotal study in the second half of calendar 2020, these findings will be used to support set-up activities.
“In the meantime, the fact that the PFS has remained robust as the analysis is extended out to the full data set gives us a great deal of additional confidence in the efficacy signal it provides.
“For CanRead More – Source
[contf]
[contfnew]

Proactiveinvestors
[contfnewc]
[contfnewc]




