Cellmid reports on results of Doherty Institute testing of serology-based COVID-19 assay
Cellmid Limited (ASX:CDY) has reported the results from testing of the Wondfo SARS-CoV-2 Antibody Tests by the Doherty Institute.
The report was produced by the Doherty Institute for the Therapeutic Goods Administration (TGA) as part of its post-market review of all serology-based COVID-19 POC tests on the ARTG (Australian Therapeutic Goods Register) to verify their ability to detect antibodies to SARS-COV-2 (the virus that causes COVID-19).
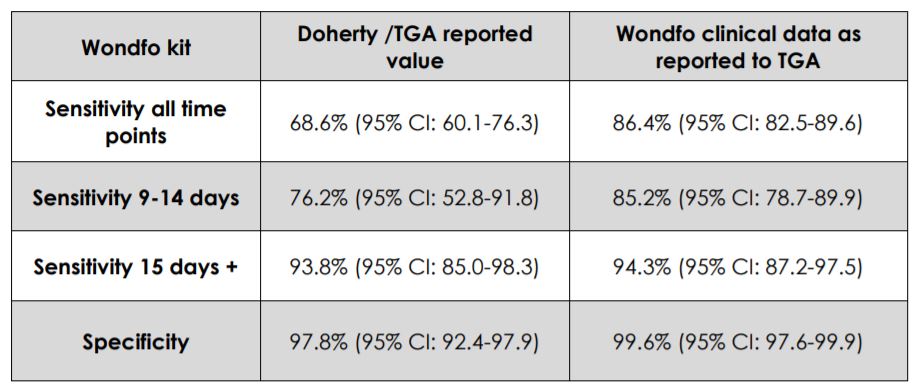
Performance of Wondfo Test in the interim Doherty Report and manufacturers specifications
Cellmid CEO Maria Halasz said: “Whilst it is noted that there are still seventeen other tests listed on the ARTG to be reviewed, we are very pleased to see that the results of the review outlined in the interim Doherty Report make the Wondfo Test one of the best performing of the five antibody tests reviewed to date across all specificity and sensitivity metrics.
“The testing showed performance results which are consistent with the manufacturers claims in the meaningful period of 14+ days following onset of symptoms, when antibodies are expected to be present in most patients.”
Accuracy of serology-based assays[hhmc]
In its commentary on the interim Doherty Report the TGA state: “As yet not enough is known about the adequacy of the COVID-19 immune response or duration of immunity.
“Whether there is a role for these tests in determining immunity for return to work purposes or for population-level surveillance remains to be seen.”
However, there is a growing body of evidence that serology-based assays increase the accuracy and speed of diagnosis when combined with PCR methods and assessment of clinical symptoms.
Furthermore, a population study by the Spanish government used a poRead More – Source
[contf]
[contfnew]

Proactiveinvestors
[contfnewc]
[contfnewc]




